 Research Applications
Research Applications
- in vitro assays
- tissue engineering/ regenerative medicine
- in vivo studies

Collagen is one of the most abundant proteins in the human body and serves as the main structural protein of the extracellular matrix (ECM) in connective tissues such as ligaments, tendons, cartilage and skin. Collagen is continuously produced and replaced to combat the breakdown and loss of connective tissue from daily wear and tear, injury or disease. However, as we age, that natural regenerative ability diminishes and our bodies are able to produce less and less collagen. Thus, highly purified collagen extracted from animal tissues in its native state can be used in clinical applications and in various formulations to replace the body’s native collagen.
Collagen serves several functions in the human body, maintainig the integrity and elasticity of the body’s connective tissues and thus supporting the form and function of the skin, cartilage and bones. The protein exists in a triple-helix formation that confers the necessary mechanical resistance to tensile forces and provides the capacity to bind a variety of macromolecules that form the extracellular matrix network. It is naturally assembled in several configurations, ranging from networks to fibrils, all of which serve various structural, mechanical and organizational roles to define local tissue architecture and influence cellular behavior.
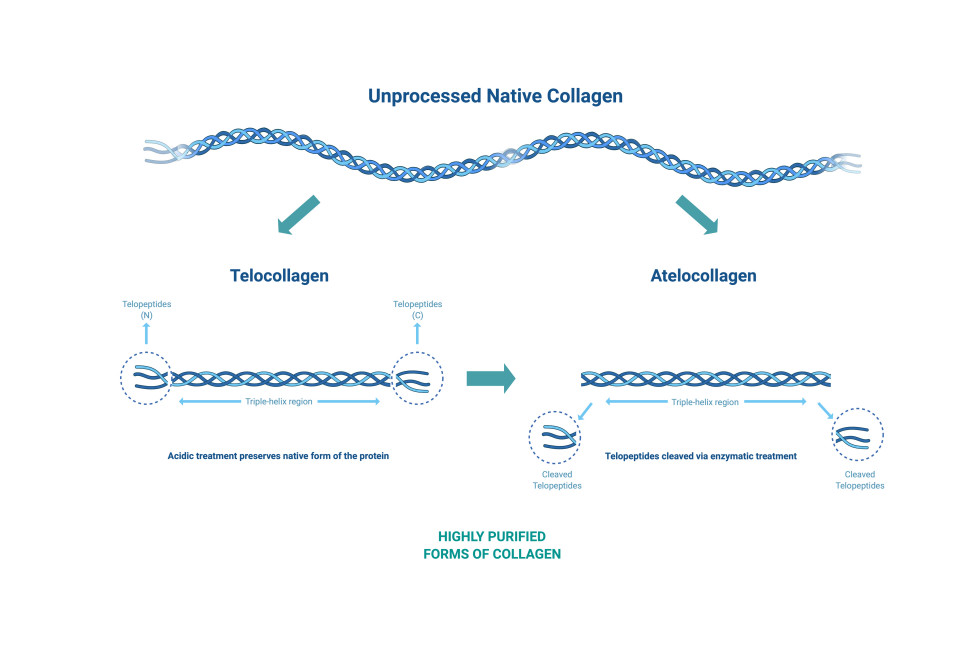
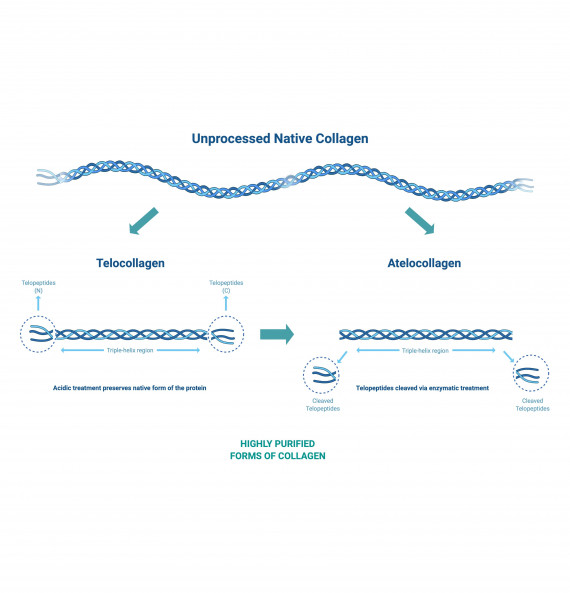
Acid extraction processes can be used to produce collagen from animal tissue maintaining the telopeptide ends of the collagen chains. Enzyme-based extraction can remove the telopeptides resulting in atelocollagen that exhibits reduced immunogenicity.
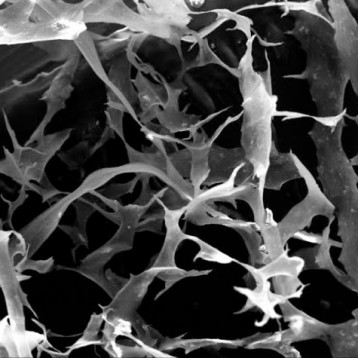
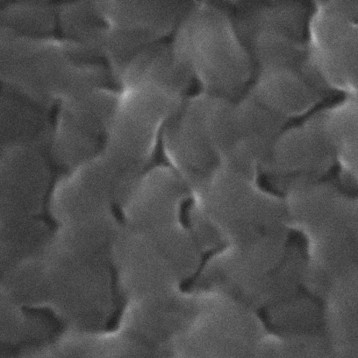
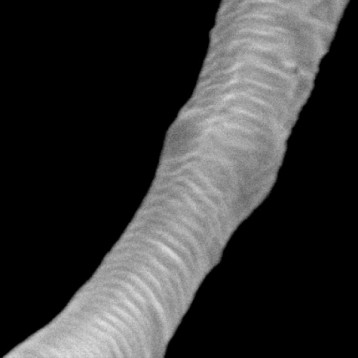
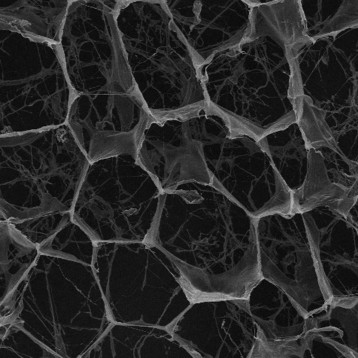

Figure shows highly purified atelocollagen product, both in lyophilized form (A) and in solution (B), without the presence of other protein impurities or extraction by-products.
Representative images and data are the property of Promed Bioscience Ltd, and cannot be reproduced without the express permission of the company.